Key Inclusions
- An executive summary of the insights captured during our research. It offers a high-level view on the current state of long-acting drug delivery technologies and services market and its likely evolution in the mid-long term.
- A general overview of long-acting drug delivery and the different types of long acting drugs delivery systems. Additionally, it highlights the underlying principles and strategies associated with long-acting drug delivery.
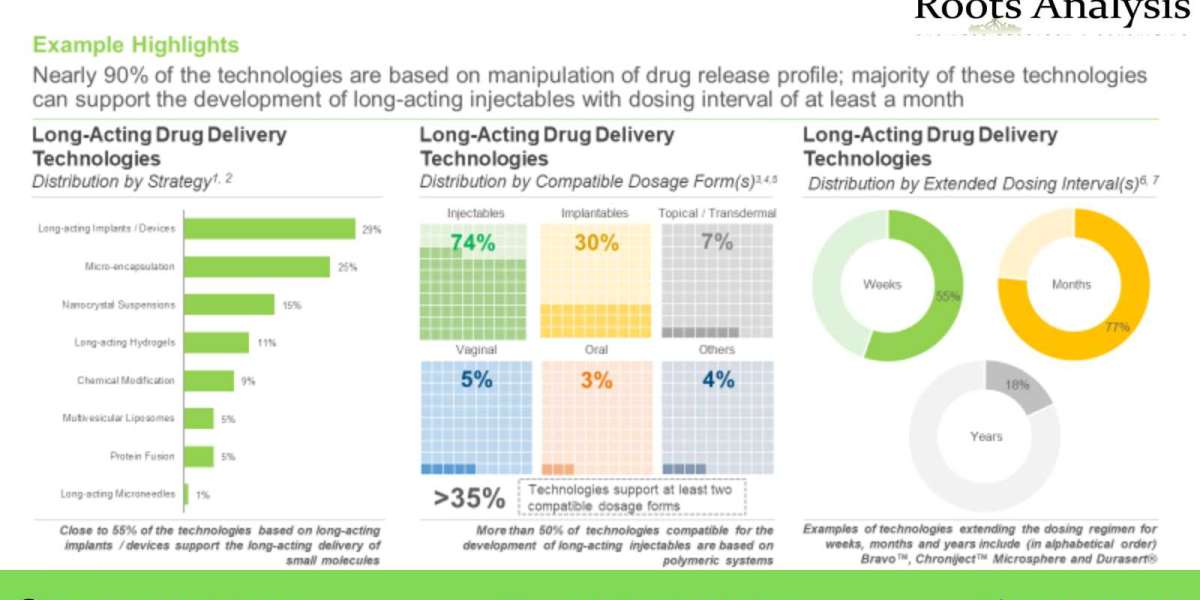
- A detailed assessment of the current technology landscape of long-acting drug delivery technologies, based on several relevant parameters, such as principle (manipulation of drug release from delivery systems and manipulation of in vivo clearance), strategy (chemical modification, micro-encapsulation, long-acting hydrogels, long-acting implants, long-acting microneedles, multivesicular liposomes, nanocrystal suspensions and protein fusion), type of material used (polymer based and non-polymer based), compatible dosage form(s) (injectables, implantables, oral, topical / transdermal, vaginal and other dosage forms), extended dosing interval(s) (weeks, months and years), type of molecule(s) delivered (biologics, small molecules and other molecules), highest phase of development (preclinical, clinical and approved), number of approved drugs and therapeutic area(s) (oncological disorders, ophthalmological disorders, neurological disorders, opioid use disorders / pain, women health, metabolic disorders, infectious diseases, cardiovascular disorders, autoimmune disorders, immunological disorders and others). In addition, the chapter features information on various technology developers, along with analysis based on multiple parameters, such as their year of establishment, company size, location of headquarters and most active players (in terms of number of technologies developed).
- A detailed assessment of the overall service providers landscape of the companies offering contract services related to long-acting drug delivery, based on several relevant parameters, such as year of establishment, company size (in terms of number of employees), location of headquarters, location of facilities, type of service provider(s) (contract development organization, contract manufacturing organization and contract development and manufacturing organization), scale of operation (preclinical, clinical and commercial), compatible dosage form(s) (injectables, implantables, oral, topical / transdermal, vaginal and other dosage forms), service(s) offered (product development, process development / pre-formulation, analytical method development, formulation development, manufacturing, technology transfer, stability studies, feasibility studies, scale-up, regulatory support and others) and type of molecule(s) supported (biologics, small molecules and other molecules).
- A technology competitiveness analysis of long-acting drug delivery technologies based on developer power (in terms of the experience of the developer) and technology strength (in terms of principle, strategy, type of material used, compatible dosage form(s), extended dosing interval(s), type of molecule(s) delivered, highest phase of development and therapeutic area(s)).
- A company competitiveness analysis of long-acting drug delivery service providers based on company strength (in terms of years of experience and company size) and service strength (in terms of number of technology platform(s), type of service provider(s), scale of operation, compatible dosage form(s), service(s) offered and type of molecule(s) supported).
- Elaborate profiles of prominent players developing technologies and offering services in the domain of long-acting drug delivery, located across North America, Europe and Asia-Pacific (shortlisted based on a proprietary criterion). Each profile features a brief overview of the company, details related to its technology portfolio, service portfolio, recent developments and an informed future outlook.
- A detailed analysis of the recent partnerships inked between stakeholders engaged in this domain, since 2018, covering acquisitions, manufacturing agreements, product development and commercialization agreements, service alliance, technology licensing agreements, and other relevant agreements.
- A detailed analysis of over 570 peer-reviewed, scientific articles focused on research related to long-acting drug delivery, based on several relevant parameters, such as year of publication, type of publication and popular keywords. The chapter also highlights the top journals, top publishers, top copyright holders and key funding institutes (in terms of number of articles published).
- An in-depth analysis of various patents that have been filed / granted related to long-acting drug delivery, since 2018, taking into consideration parameters, such as publication year, geographical region, CPC symbols, leading players (in terms of number of patents filled / granted) and type of organization. In addition, the chapter includes a detailed patent benchmarking and an insightful valuation analysis, highlighting the leading patents (in terms of number of citations).
- A detailed review of academic grants that have been awarded to various research institutes for projects focused on long-acting drug delivery, since 2018, based on several parameters, such as year of grant award, amount awarded, support period, type of funding institute center, type of grant application, purpose of grant award, activity code, NIH spending category, study section involved, popular NIH departments (in terms of number of grants) and type of recipient organization. Further, the chapter also highlights the prominent program officers (in terms of number of grants) and popular recipient organizations (in terms of number of grants and amount awarded).
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
- Principle
- Manipulation of drug release from delivery systems
- Manipulation of in vivo clearance
- Strategy
- Chemical Modification
- Micro-encapsulation
- Long-Acting Hydrogels
- Long-Acting Implants
- Long-Acting Microneedles
- Multivesicular Liposomes
- Nanocrystal Suspensions
- Protein Fusion
- Compatible Dosage Form
- Injectables
- Implantables
- Oral Dosage Forms
- Topical / Transdermal Dosage Forms
- Vaginal Dosage Forms
- Other Dosage Forms
- Type of Molecules Delivered
- Small Molecules
- Biologics
- Other Molecules
- Type of Material Used
- Polymer based
- Non-polymer based
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and North Africa
Frequently Asked Questions
- What are the major factors driving the long-acting drug delivery market?
- How many long-acting drug delivery technologies, compatible with long-acting injectables, are available in the drug delivery market?
- How many long-acting drug delivery technologies demonstrate extended dosage regimen of at least a year?
- How many long-acting drug delivery technologies demonstrate extended dosage regimen of at least a year?
- How many contract service providers possess technology transfer capabilities related to long-acting drug delivery?
- What are the partnership and collaboration trends in the long-acting drug delivery domain?
- What is the current patent landscape of long-acting drug delivery market?
- Which segment, in terms of strategy, accounts for the largest share in the global long-acting drug delivery technologies market?
- Which geography is expected to witness the highest growth rate in the long-acting drug delivery services market?
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/long-acting-drug-delivery-market.html
You may also be interested in the following titles:
Digital Biomanufacturing Market |
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415