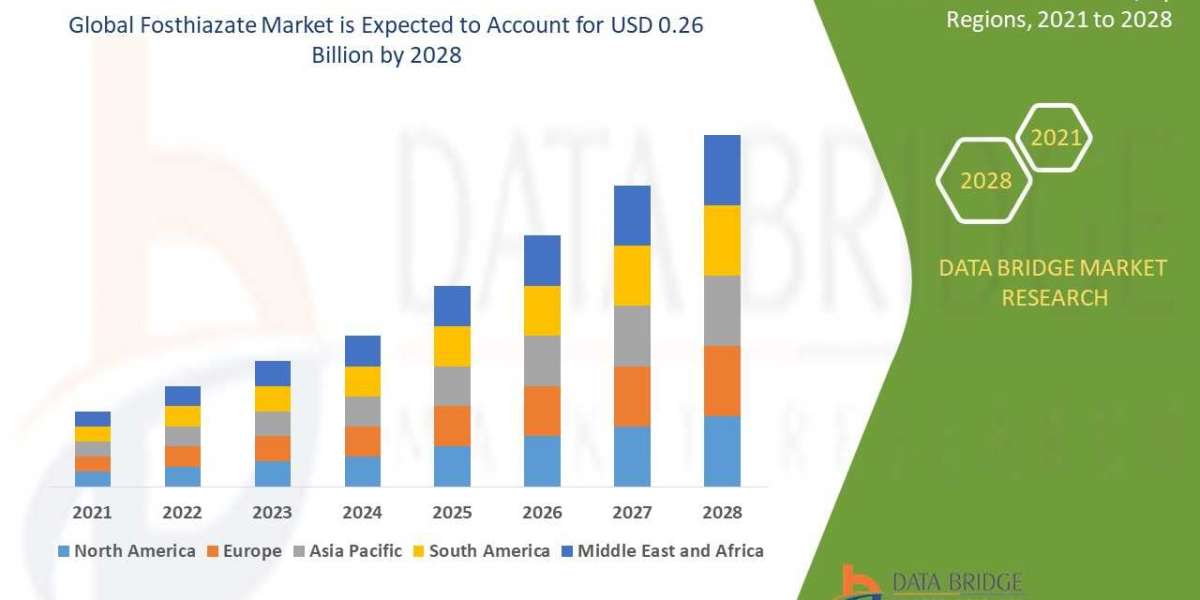
Chemical reactors are pieces of equipment in which reactants are converted into a desired product. They are vital for industrial processes to function efficiently and safely.
There are several ways to increase the productivity of a chemical reactor. Some methods involve manipulating the concentration of the reaction, while others affect the temperature.
Semi-batch Reactors
A semi-batch reactor is a type of reaction vessel that has a continuous flow of another reagent while the first reactant is being used up. This allows the process to run much more efficiently than a traditional batch reactor. It also provides the flexibility to use different amounts of each reagent, which can be more economical.
While many simulation studies on off-line optimization of batch reactors can be found, applications to laboratory or production scale reactors are not as common. However, Toulouse et al. [5] have simulated the operation of an industrial semi-batch reactor with a strongly exothermic polymerization reaction and optimized the process to reduce its batch time. The optimization was based on an accurate model of the process, the design of a reduced model for off-line optimization, and calculation of optimal trajectories in which various operational as well as quality and safety constraints were taken into account.
In their study, the authors found that the batch length of the polystyrene polymerization with 2, 2 azobisisobutyronitrile (AIBN) as initiator could be significantly reduced by decreasing the initial monomer concentration Ci0 and increasing the reaction temperature Tr. These changes lead to shorter batch times without compromising the monomer conversion.
The underlying kinetic model is described in detail using a plausible mechanism and can be solved by means of the dynamic optimization method available in gPROMS. The optimal trajectories are verified experimentally with an industrial reactor.
Continuous Reactors
While batch reactors still dominate fine and complex chemical manufacturing, continuous processes are increasingly being used in new high value products. These processes use continuous flow reactors that eliminate the wasted time of daily start-ups, shutdowns, and empty-fill / heat up cycles. This enables manufacturers to increase productivity by reducing cycle times.
Continuous reaction systems have far more effective mass transfer and heat transfer capabilities than batch, allowing for much faster reaction rates. The small size of continuous reactors also enables much better concentration profiles with more separation between reactants and products. This can lead to significant improvements in product yield and quality.
Typical CSTRs (continuous stirred tank reactors) are able to increase specific reaction rates by up to 8-fold compared to batch – with the same reaction conditions. However, the residence time of a single CSTR is limited by the mixing impeller design and can be difficult to control, leading to dead zones. This can be solved by using a cascade of CSTRs to ensure a consistent residence time - although this is more costly in terms of capital investment and utilities.
Alternatively, oscillatory baffled continuous reactors rely on a combination of static mixing with cycling of the flow direction to provide efficient mixing without dead zones. The process chemistry may require the use of solid catalysts in a liquid reaction medium, or the chemistry may be homogeneous (catalyst and reaction medium are in the same liquid phase). In such cases, an agitation system that allows for efficient dispersing of the solids can be used.
Oscillatory Flow Reactors
Oscillatory flow reactors produce intense mixing and enhanced mass and heat transfer. They can be operated continuously or batchwise and are flexible for the production of multiple products and feedstocks. This type of reactor is particularly well-suited for endothermic reactions such as biofuel production.
In contrast to conventional CSTRs and tubular reactors that rely on stirring mechanisms or turbulent flow conditions for mixing, OBRs use oscillations to create vortices that effectively act as many small-scale CSTRs connected in series (figure 2). This results in each inter-baffle zone acting as an individual CSTR, allowing long residence time processes to be achieved in reduced length reactors.
For example, Lodha and Jachuz used an oscillatory flow reactor to produce eco-friendly biodiesel from canola oil with sodium hydroxide as a catalyst. The reaction was completed in less than 28 s, with a conversion of 99.5% and a yield of 10 tons/day. buy reactors at most affordable price like Surplusrecord.
Another option to increase productivity is through the use of microreactors. These devices are designed to perform a wide range of chemical reactions, including hydrogen production and ethylene partial oxidation. They are distinguished by their small channel diameter, which allows for high mass transfer rates and efficient heat management.
Microreactors
One of the latest innovations in flow chemistry is the microreactor. Microreactors are small-scale reactors that can be used for continuous production or as a tool for scale-up of single or multiphase reactions. They have an array of benefits over traditional tubular and batch reactors, including better mixing, lower energy consumption, smaller volume, and more controllable temperature.
These microreactors are also more cost-effective than traditional batch and tubular used reactors because they are smaller and require less space, and they can be built from cheaper materials. Furthermore, they can increase productivity by allowing for faster reactions and higher yields. Additionally, they are more flexible because they can be used for both endothermic and exothermic reactions.
The process of making chemicals with a microreactor is relatively simple. For example, a company called Last Energy has developed a prototype reactor that can produce fuel and hydrogen from water. The reactor is housed in an edgy cube covered in reflective metal and is capable of producing up to 1,000 watts of electricity per hour.
Despite the advantages of microreactors, there are still some challenges. For example, they can be difficult to clean and can suffer from corrosion. Another challenge is that the reaction conditions can change with time, so it is important to monitor them frequently and make changes accordingly. Furthermore, it is also important to consider how to handle the microreactor in case of an accident or an exothermic runaway reaction.